Fireworks?

Coffee art?

A flower blooming?

Nope, we’re diving into something even cooler (or should we say hotter?). Imagine watching chemistry come alive, not with fancy experiments, but with a thermal camera that shows heat as it happens!
Let’s take a closer look at what happens when sodium hydroxide (NaOH) meets water, why it’s so fascinating, and how you can see other exothermic reactions in action. Ready? Let’s heat things up!
What's an Exothermic Reaction?
An exothermic reaction is just a fancy way of saying, "This makes things hot!" It's a chemical reaction that releases heat, causing the temperature around it to rise.
Here’s a classic example: when you add NaOH (sodium hydroxide) to water, something magical happens. The NaOH dissolves and releases a bunch of heat. To the naked eye, it looks like plain water with some stuff added. But with a thermal camera? It’s a whole different story.


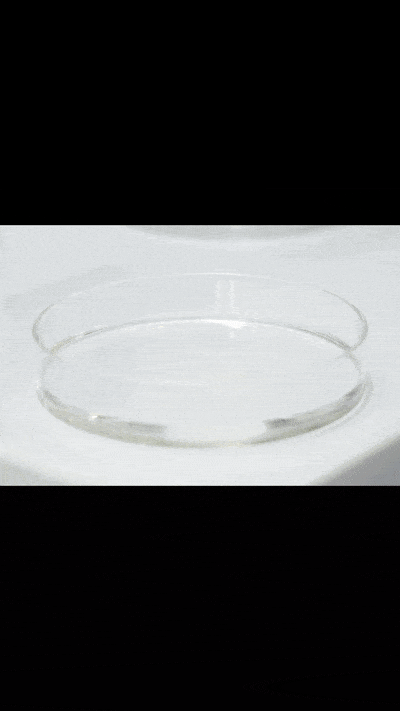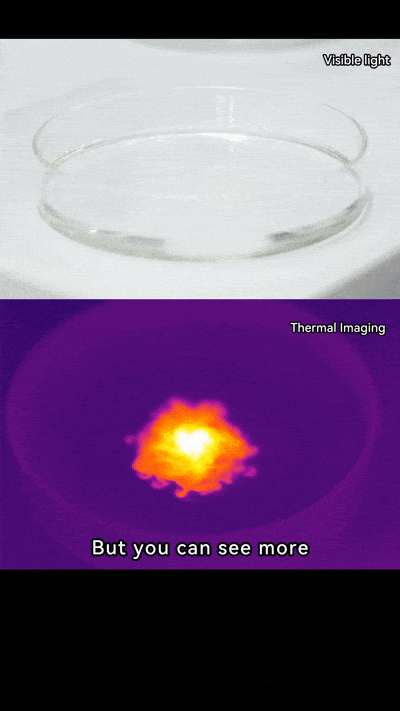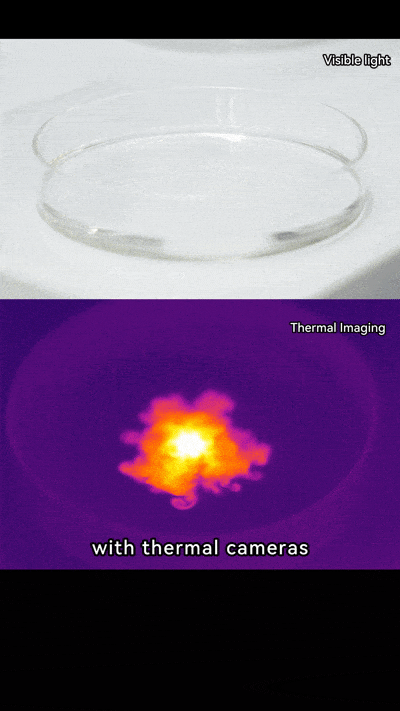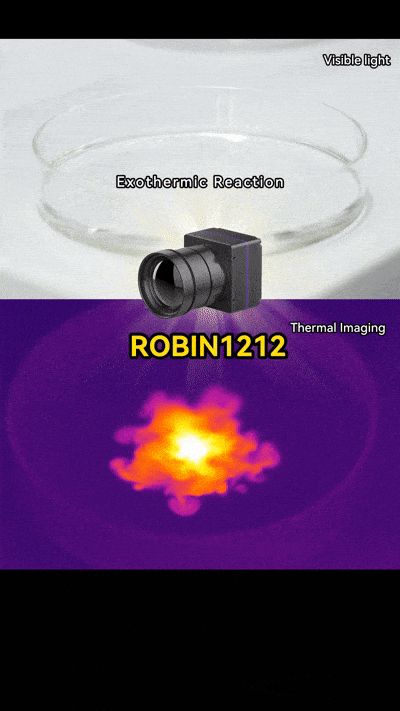
Thermal imaging cameras let us see heat in action. They detect infrared energy and show it as colors, turning invisible heat into something we can watch.
When you add NaOH to water, the heat spreads quickly. On a thermal camera, it looks like a flower opening or even a mini firework going off. Pretty neat, right? It’s not just science; it’s like art created by chemistry.










